Lifespan Neuromodulation of Cognition (LINE)
Research Scientists
Martin Dahl
Ulman Lindenberger
Agnieszka Kulesza
MRI-Indexed Locus Coeruleus Integrity is Linked to Neural Pathology and Cognition
Pupil-Indexed Neuromodulation is Associated With Rhythmic Neural Activity and Attention
Neuromodulators are a group of chemicals that are synthesized in small subcortical nuclei, from which they are released via far-reaching fibers throughout the brain. Neuromodulator release shapes the efficacy of synaptic transmission in its target regions and thus has a profound influence on cognitive processes, such as attention and memory. Furthermore, neuromodulatory nuclei are susceptible to degeneration in aging and disease, thereby contributing to senescent cognitive decline. Continuing a line of research initiated in the RHYME project, LINE uses a multimodal approach to investigate structural and functional age differences and age changes in neuromodulation and their associations with normal and pathological forms of cognitive aging. Conceptually, the project aims at a comprehensive understanding of the role of neuromodulation in cognitive aging (Dahl, Mather, Werkle-Bergner, & Kennedy, 2022). During the reporting period, the research activities of this new project were centered on two interrelated themes.
MRI-Indexed Locus Coeruleus Integrity is Linked to Neural Pathology and Cognition
Research dissecting the brains of people who died at different ages indicates that the first signs of Alzheimer’s-related pathology, abnormally phosphorylated tau, begin to accumulate early in life in the locus coeruleus, the brain’s main source of the neuromodulator noradrenaline. For a long time, in-vivo human studies of the locus coeruleus were deemed virtually impossible due to its small size and location deep in the brainstem. However, recently developed magnetic resonance imaging (MRI) techniques have resulted in non-invasive markers for the integrity of the neuromodulatory nucleus (see Figure 1a). In earlier work within the RHYME project, we had assessed MRI-indexed locus coeruleus integrity in the Berlin Aging Study II (cf. Berlin Aging Studies project). We detected spatially confined and functionally relevant age differences in locus coeruleus integrity. A follow-up study in the same sample (Bachman et al., 2021) demonstrated an association of higher locus coeruleus integrity and cortical thickness, particularly in frontoparietal cortices.
In a next step, we sought to clarify (a) whether in-vivo differences in locus coeruleus integrity are corroborated by post-mortem data and (b) whether differences in MRI-indexed locus coeruleus integrity are more indicative of healthy or pathological aging processes. In a collaboration with John M. Ringman and Mara Mather, University of Southern California, we studied participants with, or at risk for, mutations in genes causing familial Alzheimer’s disease with early onset (autosomal-dominant Alzheimer’s disease, ADAD), providing a unique window into the pathogenesis of Alzheimer’s largely disentangled from common age-related factors (Dahl, Mather, Werkle-Bergner et al., 2022). Combining high-resolution MRI with assessments of Alzheimer’s-related tau pathology, we observed lower MRI-indexed locus coeruleus integrity in symptomatic mutation carriers (see Figure 1b). Moreover, participants with lower locus coeruleus integrity showed higher cortical tau burden and lower memory performance. To corroborate our in-vivo imaging findings, we additionally analyzed post-mortem locus coeruleus specimens from a separate dataset of carriers of the same mutation and indeed found substantial locus coeruleus degeneration.
Taken together, across studies, our findings link lower locus coeruleus integrity to markers of neural and cognitive decline. Moreover, they suggest that late-life differences in locus coeruleus integrity may be indicative of pathological aging processes.

Figure 1. Locus coeruleus magnetic resonance imaging (LC-MRI) in healthy aging and neurodegenerative disease. (a) Orientation of a typical locus coeruleus-sensitive sequence covering the brainstem (left). The localization of the locus coeruleus, bordering the fourth ventricle, is highlighted in red on an axial slice of standard anatomical scan (middle, upper panel). In LC-MRI, the locus coeruleus can be detected as a cluster of bright, hyperintense voxels (middle, lower panel). (b) In neurodegenerative diseases, noradrenergic cells decline. Lower in-vivo LC-MRI contrast in autosomal-dominant Alzheimer’s disease (upper panel) corresponds to noradrenergic neurodegeneration in participants who died with the same disease-causing mutation (A431E). In particular, hematoxylin and eosin staining reveals locus coeruleus depigmentation (i.e., absence of the dark, granular neuromelanin). In addition, immunostained slides with anti-tau (AT8) show neurofibrillary tangles within noradrenergic neurons as well as the presence of tau positive threads (red).
Image: MPI for Human Development
Adapted from Dahl, Mather, & Werkle-Bergner (2022) with permission
Key Reference
Pupil-Indexed Neuromodulation is Associated With Rhythmic Neural Activity and Attention
While advances in MRI techniques allow the characterization of individual differences in the integrity of neuromodulatory nuclei, animal research indicates that behaviorally relevant stimuli elicit a burst of activity in neuromodulatory centers that facilitates their processing. Recent studies across rodents and non-human and human primates have revealed an association between luminance-independent changes in pupil dilation and neuromodulatory activity, particularly of the locus coeruleus. This suggests that pupil dilation may serve as a moment-to-moment proxy of noradrenergic activity across species.
To investigate the role of noradrenergic neuromodulation on brain dynamics and cognition, we asked younger and older participants to complete an auditory attention task while concurrently recording electroencephalography (EEG) and pupil dilation (Dahl et al., 2020). During the attention task, arousing or perceptually matched control stimuli were presented on a trial-by-trial basis to dynamically modulate arousal-related noradrenergic drive. Crucially, larger pupil dilation in response to arousing stimuli was associated with a stronger transient EEG desynchronization (alpha–beta frequency bands; 9–30 Hz), a marker for cortical excitability. This supports the hypothesis that arousal-related changes in pupil dilation and cortical excitability share a common underlying dependence on noradrenaline release. Behaviorally, noradrenergic responsiveness, as approximated by greater pupil dilation and EEG desynchronization, was linked to better attention performance across several tasks. Comparing age groups, we observed that older age was associated with reduced noradrenergic responsiveness, suggesting a role of the locus coeruleus in senescent attentional decline.
Following this empirical work, we reviewed the animal and human literature to derive a potential mechanism explaining the observed link between noradrenergic neuromodulation and cortical excitability adjustments during attention (Dahl, Mather, & Werkle-Bergner, 2022). In brief, we propose that during moments involving selective attention, the thalamus orchestrates the preferential processing of prioritized information by coordinating rhythmic neural activity within a distributed frontoparietal network (see Figure 2). The timed release of neuromodulators from subcortical structures dynamically sculpts neural synchronization in thalamocortical networks to meet current attentional demands. In particular, we posit that noradrenaline modulates the balance of cortical excitation and inhibition, as reflected by thalamocortical alpha synchronization (~8–12 Hz), and that these neuromodulatory adjustments facilitate the selective processing of prioritized information.
Taken together, we have taken a multimodal approach to demonstrate associations between locus coeruleus integrity in aging and disease and markers of neural and cognitive decline. In addition, we showed the influence of moment-to-moment changes in noradrenergic activity on cortical dynamics and cognition.
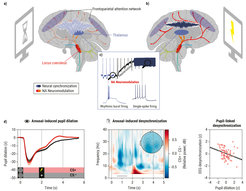
Figure 2. Locus coeruleus–noradrenergic neuromodulation shapes patterns of thalamocortical synchronization during attention. (a) Anatomically and functionally defined locus coeruleus ensembles innervate different cortical and subcortical targets (see colored cells/projections [circles/arrows] originating from the brainstem), including the thalamus and frontoparietal network (blue and gray ovals). During inattentiveness, low noradrenaline levels are linked to rhythmic burst firing in thalamic alpha generators (see c). Alpha-rhythmic activity (~8–12 Hz) in thalamocortical attention networks indicates a state of relative inhibition (see high-amplitude low-frequency oscillation [light blue waveform]). (b) Behaviorally relevant events elicit a transient increase in locus coeruleus activity. Elevated noradrenaline (NA) levels modulate cortical synchronization by shifting thalamic pacemakers from a rhythmic firing pattern to a mode of activity that allows for reliable information transfer (single-spike firing; see c). Neural activity in thalamocortical attention networks is desynchronized (dark blue), supporting the processing of attended stimuli. (c) Noradrenaline depolarizes thalamic neurons and abolishes the rhythmic burst firing that is linked to thalamocortical alpha rhythms. Sagittal brain section adapted from Patrick J. Lynch under CC BY 2.5. (d) Pupil-indexed noradrenergic neuromodulation is related to cortical low-frequency desynchronization. Left: Compared to perceptually matched control stimuli (CS–), fear-conditioned stimuli (CS+) elicit a transient pupil dilation, a marker of locus coeruleus activity. Center: Concurrent electroencephalography recordings reveal an arousal-related alpha–beta desynchronization at posterior electrodes (topography shows averaged activity between 0.5–1.5 seconds and 8–20 Hz [gray horizontal bar]). Right: Larger pupil dilation is associated with more alpha–beta desynchronization (i.e., more negative values), indicating an association between proxies of noradrenergic neuromodulation and cortical synchronization.
Image: MPI for Human Development
Adapted from Dahl, Mather, & Werkle-Bergner (2022) with permission
Key Reference
Outlook
Our current work extends these lines of research by combining longitudinal high-resolution MRI of the dopaminergic substantia nigra–ventral tegmental area and the noradrenergic locus coeruleus in younger and older adults (Dahl, Bachman, et al., 2023). We find that dopaminergic and noradrenergic integrity are differentially associated with individual differences in older adults’ memory performance. Whereas higher noradrenergic integrity is related to better episodic memory across several memory tasks, higher dopaminergic integrity is linked to better working memory. Longitudinally, we find that older age is associated with more negative change in both neuromodulatory nuclei from first to second measurement occasion (mean distance = 1.9 years). We found that changes in locus coeruleus integrity reliably predict future episodic memory performance at a third occasion (mean distance to second occasion = 2.9 years). In a series of future studies (Dissertation Agnieszka Kulesza), we plan to combine cognitive and MRI data of the longitudinal studies AKTIV (cf. Plasticity project) and BASE-II to investigate within-person changes in neuromodulatory integrity and explore links to blood-based biomarkers for Alzheimer’s disease.
Key Reference

![Figure 2. Locus coeruleus–noradrenergic neuromodulation shapes patterns of thalamocortical synchronization during attention. (a) Anatomically and functionally defined locus coeruleus ensembles innervate different cortical and subcortical targets (see colored cells/projections [circles/arrows] originating from the brainstem), including the thalamus and frontoparietal network (blue and gray ovals). During inattentiveness, low noradrenaline levels are linked to rhythmic burst firing in thalamic alpha generators (see c). Alpha-rhythmic activity (~8–12 Hz) in thalamocortical attention networks indicates a state of relative inhibition (see high-amplitude low-frequency oscillation [light blue waveform]). (b) Behaviorally relevant events elicit a transient increase in locus coeruleus activity. Elevated noradrenaline (NA) levels modulate cortical synchronization by shifting thalamic pacemakers from a rhythmic firing pattern to a mode of activity that allows for reliable information transfer (single-spike firing; see c). Neural activity in thalamocortical attention networks is desynchronized (dark blue), supporting the processing of attended stimuli. (c) Noradrenaline depolarizes thalamic neurons and abolishes the rhythmic burst firing that is linked to thalamocortical alpha rhythms. Sagittal brain section adapted from Patrick J. Lynch under CC BY 2.5. (d) Pupil-indexed noradrenergic neuromodulation is related to cortical low-frequency desynchronization. Left: Compared to perceptually matched control stimuli (CS–), fear-conditioned stimuli (CS+) elicit a transient pupil dilation, a marker of locus coeruleus activity. Center: Concurrent electroencephalography recordings reveal an arousal-related alpha–beta desynchronization at posterior electrodes (topography shows averaged activity between 0.5–1.5 seconds and 8–20 Hz [gray horizontal bar]). Right: Larger pupil dilation is associated with more alpha–beta desynchronization (i.e., more negative values), indicating an association between proxies of noradrenergic neuromodulation and cortical synchronization. Locus coeruleus–noradrenergic neuromodulation shapes patterns of thalamocortical synchronization during attention](/49345/original-1687867768.jpg?t=eyJ3aWR0aCI6ODQ4LCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6NDkzNDV9--6a930f7d91fddc8bd96c0aaded381ac868ae1c3f)