The Berlin Aging Studies (BASE & BASE-II)
Research Scientists
Julia Delius
Sandra Düzel (until 08/2022)
Caroline Beese (until 7/2021)
Andreas Brandmaier
Maike Kleemeyer (until 03/2021; now Research Data Management)
Ylva Köhncke (until 12/2022)
Ulman Lindenberger
Georg G. Wagner (Max Planck Fellow)
The Berlin Aging Study (BASE)
The Berlin Aging Study II (BASE-II)
In the course of the 20th century, average life expectancy almost doubled. What do these added years mean in terms of functional capacity and quality of life? And how do the last years and months preceding death in old age differ from the years before? For more than 3 decades, the Berlin Aging Studies have helped to answer questions of this sort. Members of the Center have been investigating changes in cognition and other aspects of behavior in the context of the Berlin Aging Study (BASE; Baltes & Mayer, 1999; Lindenberger et al., 2010) and, later, the Berlin Aging Study II (BASE-II; Demuth et al., 2021). Both studies are collaborative and multidisciplinary, involving researchers from institutions inside and outside Berlin. The Berlin Aging Studies also formed part of the Lifebrain consortium, which was funded under Horizon 2020, the EU Framework Programme for Research and Innovation.
The Berlin Aging Study (BASE)
More than 30 years ago, 516 Berliners aged 70 years and over participated in the first measurement occasion of BASE. Longitudinal data are available for eight measurement occasions spanning more than 18 years, and mortality-related information was updated at regular intervals until the last participant passed away in 2019 (see below for an overview). We are happy to note that BASE data continue to be the source of original publications on individual differences in late-life development (e.g., Wahl et al., 2022). In particular, the availability of similar or identical measures in BASE and BASE-II has permitted the investigation of cohort differences in various aspects of normal aging, such as cognitive decline (Gerstorf et al., 2023) and perceptions of time passing (Löckenhoff et al., 2022).
In line with the Open Science policy of the Institute, access to the BASE data for the academic community will be facilitated further upon its transferal to the Research Data Center at the Leibniz Institute for Psychology (ZPID) toward the end of 2023. Julia Delius and Maike Kleemeyer, a former project member who was appointed the MPI for Human Development’s research data management coordinator in March 2021, are currently working on this project (see Research Data Management).
Key Reference
The Berlin Aging Study II (BASE-II)
The central objective of the multidisciplinary and multi-institutional longitudinal Berlin Aging Study II (BASE-II; see overview below) is to promote a better understanding of individual differences and trajectories in cognitive, psychosocial, and physical functioning by integrating multidisciplinary perspectives and data. In the following, we showcase three paper projects from the reporting period.
Latent Factors of Gray Matter Integrity Correlate With Episodic Memory Ability in Old Age
Maintained structural integrity of hippocampal and cortical gray matter may explain why some older adults show more preserved episodic memory than others. However, viable measurement models for estimating individual differences in gray matter structural integrity have been lacking; instead, most studies have relied on fallible single indicators. To ameliorate this shortcoming, Köhncke et al. (2021) introduced multitrait–multimethod methodology to the cognitive neuroscience of aging, with the goal of more reliably capturing individual differences in gray matter integrity. We analyzed data from 1522 BASE-II participants aged 60 to 88 years, including 333 participants who underwent magnetic resonance imaging. We were able to establish structural integrity latent factors for hippocampus, parahippocampal gyrus, prefrontal cortex, and precuneus respectively, each of which express the common variance of voxel-based morphometry, mean diffusivity, and magnetization transfer ratio. Except for precuneus, the structural integrity factors showed reliable positive associations with a latent factor of episodic memory ability (see Figure 1). For hippocampal and parahippocampal regions, associations persisted after controlling for age, sex, and education. In line with theoretical propositions (Nyberg & Lindenberger, 2020), these results show that episodic memory ability in old age benefits from maintained structural integrity of hippocampus and parahippocampal gyrus. We conclude that multimodal factors of structural brain integrity help to capture common variance in performance-relevant properties of gray matter, and underscore the need to arrive at a better understanding of the physiological factors that contribute to this common variance.
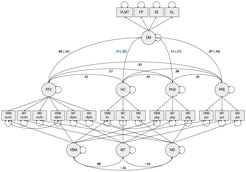
Figure 1. Latent factors of gray-matter integrity are correlated to episodic memory in old age. For each region of interest (PFC, HC, PHG, PRE), the model expresses variance common to three different structural imaging modalities as a latent gray-matter integrity factor by separating it from method-specific factors (V, MT, MD), and residual variance (double-headed arrows at the observed variables depicted by squares), and links the latent integrity factors to episodic memory. Circles depict latent variables, squares depict observed variables. Double-headed arrows are covariances, or, if self-referential, variances. Single-headed arrows are directed effects. Numbers next to the arrows are correlations (standardized covariance estimates). Correlations not in parentheses are from the final model in which all latent factors are regressed on age, sex, and education; correlations in parentheses refer to correlations without controlling for age, sex, and education. Correlations written in blue font are reliably different from zero (p < .05). Structural imaging modalities: VBM = VBM-derived gray matter probability, MT = magnetization transfer ratio, MD = mean diffusivity. Regions of interest: mofc = medio-orbitofrontal cortex, dlpfc = dorsolateral prefrontal cortex, hc = hippocampus, phg = parahippocampal gyrus. Cognitive tasks: VLMT = verbal learning and memory test, FP = face-profession task, SE = scene encoding task, OL = object location task. EM = episodic memory. We fitted the model (including covariates age, sex, education, not shown in the diagram) to data from 1521 individuals aged 60 to 88 years, assuming data in MR-derived variables to be missing at random. Fit indices: CFI = .948, RMSEA = .027 (CI .023–.031), SRMR = .047.
Image: Ylva Köhncke / MPI for Human Development
Original image licensed under CC BY 4.0
The Association Between Dopamine Integrity and Working Memory in Old Age
Dopamine integrity has been suggested as a potential cause of individual differences in working memory performance among older adults. However, identifying specific dopaminergic pathways that give rise to this variation has proven difficult. Using BASE-II data, Karalija et al. (2021) assessed 61 single-nucleotide polymorphisms, located in or adjacent to various dopamine-related genes, for their links to working memory performance. Least Absolute Shrinkage and Selection Operator (LASSO) regression was conducted to estimate associations between polymorphisms and working memory. Rs40184 in the DA transporter gene SLC6A3 showed allelic group differences in working memory, with T-carriers performing better than C homozygotes. This finding was replicated in an independent sample from the Cognition, Brain, and Aging study (COBRA) in Sweden (baseline: n = 181, ages: 64–68 years; 5-year follow-up: n = 129). In COBRA, in vivo dopamine integrity was measured with 11C-raclopride using positron emission tomography. Notably, working memory as well as in vivo dopamine integrity was higher for rs40184 T-carriers both at baseline and at the 5-year follow-up. Our findings indicate that individual differences in dopamine transporter function contribute to differences in working memory performance in old age, presumably by regulating dopamine availability.
Daily Fluctuations in Behavior in BASE-II During the COVID-19 Pandemic
The outbreak of the COVID-19 pandemic resulted in a public health emergency that posed particular threats to older adults’ physical and psychological well-being. Numerous preventive measures were geared towards protecting older adults, and massively affected their daily lives. Due to physical distancing and contact reductions, older adults had substantially fewer social interactions, which might have put them at risk of experiencing loneliness and social isolation. Similarly, older adults’ own awareness that the pandemic posed a disproportionate threat to their lives might have increased daily stress, anxiety, and negative affect. To investigate these issues in greater detail, we conducted the CorAge study as a satellite study within BASE-II (see Figure 2): 140 older adults aged 67 to 88 years from the BASE-II participant pool were asked to respond to six questionnaires on their smartphone at set times of day. On average, participants provided a total of 39 responses over 7 days. In an initial analysis (Potter et al., 2023), we examined health sensitivity, defined as the association between self-reported health and affect, and found that older adults showed greater health sensitivity in moments when they perceived an elevated risk of contracting COVID-19. Results document the influence of the COVID-19 pandemic on older adults’ emotional experiences, and will be followed up longitudinally.

Image: Johanna Drewelies / MPI for Human Development
Original image licensed under CC BY 4.0
Key Reference
Study Overviews
Overview of the Berlin Aging Study (BASE) – www.base-berlin.mpg.de
The multidisciplinary Berlin Aging Study (BASE), initially directed by the late Paul B. Baltes and Karl Ulrich Mayer, was started in 1989. Ulman Lindenberger is the current BASE speaker. The study spans eight measurement occasions spaced over 18 years. Its distinguishing features include (1) a focus on the very old (70–100+ years); (2) a locally representative sample, stratified by age and sex; and (3) a broad-based interdisciplinarity (originally involving two research units from the Freie Universität Berlin, Internal Medicine and Psychiatry, and two from this Institute, Sociology and Psychology). In addition to discipline-specific topics, four integrative theoretical orientations guide the study: (1) differential aging, (2) continuity versus discontinuity of aging, (3) range and limits of plasticity and reserve capacity, and (4) aging as a systemic phenomenon.
The initial focus of BASE (1990–1993) was to obtain a heterogeneous sample, stratified by age and sex, of individuals from the western districts of Berlin aged 70 to 100+ years. A core sample of 516 men and women completed the Intensive Protocol comprising detailed measures from all four participating disciplines. Seven longitudinal follow-ups involving different depths of assessment were completed at approximately 2-yearly intervals. Details of the research design and assessment protocols can be found on the BASE website. The core sample formed the basis of the analyses reported in two monographs (see Baltes & Mayer, 1999; Lindenberger et al., 2010). Current work uses the longitudinal data to address issues such as variability and change, mortality prediction, self-related change, and genetic and socioeconomic predictors of cognitive change.
The Berlin Aging Study: International Research Group
| Julia A. M. Delius | MPI for Human Development, Berlin, Germany |
| Alexandra M. Freund | University of Zurich, Switzerland |
| Denis Gerstorf | Humboldt-Universität zu Berlin, Germany |
| Paolo Ghisletta | University of Geneva, Switzerland |
| Christiane Hoppmann | The University of British Columbia, Vancouver, Canada |
| Ulman Lindenberger | MPI for Human Development, Berlin, Germany (Speaker) |
| Nilam Ram | Stanford University, USA |
| Jacqui Smith | University of Michigan, Ann Arbor, USA (Co-Speaker) |
| Ursula M. Staudinger | Technische Universität Dresden, Germany |
| Elisabeth Steinhagen-Thiessen | Charité Universitätsmedizin Berlin, Germany |
| Gert G. Wagner | MPI for Human Development, Berlin, Germany (Max Planck Fellow) |
Overview of the Berlin Aging Study II (BASE-II) – www.base2.mpg.de
BASE-II follows a longitudinal design. At the first wave of measurements (T1), the BASE-II sample consisted of 1,600 participants aged 60 to 80 years and 600 individuals aged 20 to 35 years. Data collection of the first wave was completed in 2014. In close collaboration with Simone Kühn of the Lise Meitner Group for Environmental Neuroscience, eligible BASE-II participants (n = 445) were additionally invited for a structural magnetic resonance imaging (MRI) assessment of the brain, comprising T1-weighted imaging, resting state data, diffusion tensor imaging, and high-resolution imaging of the hippocampus. In 2015, this MR subsample was re-invited again for another wave of cognitive and psychosocial assessments and a second MRI session (n = 327). In November 2017, the older cohort of 1,600 men and women from the original BASE-II sample was re-invited in the context of the project Sex- and Gender-Sensitive Prevention of Cardiovascular and Metabolic Disease in Older Adults in Germany (GendAge, funded by the Federal Ministry of Education and Research). GendAge includes most of the medical and biological assessments of T1, along with a third wave of cognitive and psychosocial assessments. In addition, accelerometers are used to track participants’ physical activity and sleep for a week.
In 2021 we received funding from the VW Stiftung for Dynamics of Daily-Life Adaption in the Corona Crisis Among Older Adults (CorAge), allowing us to investigate the effects of COVID-19, and of COVID-19 restrictions, on psychosocial, cognitive, and medical markers assessed 7 times daily across 7 days. A total of 104 older participants (41% female) from BASE-II with an average age of 76.4 years provided daily data on psychosocial, cognitive, and medical variables. In 2021, we also started re-inviting MR-eligible older participants to a third wave to re-assess structural MR parameters. Finally, in September 2022, we launched another cognitive and medical assessment of the remaining 1,800 younger and older BASE-II participants. This data collection is ongoing until the end of 2023.
The Berlin Aging Study II: Steering Committee
| Ulman Lindenberger | MPI for Human Development, Berlin, Germany |
| Johanna Drewelies | MPI for Human Development, Berlin, Germany |
| Sandra Düzel | MPI for Human Development, Berlin, Germany |
| Simone Kühn | MPI for Human Development, Berlin, Germany |
| Gert G. Wagner | MPI for Human Development, Berlin, Germany (Max Planck Fellow) |
| Elisabeth Steinhagen-Thiessen | Charité Universitätsmedizin, Berlin, Germany |
| Ilja Demuth | Charité Universitätsmedizin, Berlin, Germany |
| Lars Bertram | Universität zu Lübeck, Germany |
| Graham Pawelec | University of Tübingen, Germany |
| Arno Villringer | MPI for Human Cognitive and Brain Sciences, Leipzig, Germany |
| Stefan Liebig | Socio-Economic Panel (SOEP) at the German Institute for Economic Research (DIW Berlin), Germany |
| Ludmila Müller (Coordinator) | MPI for Human Development, Berlin, Germany |
Lifebrain – www.lifebrain.uio.no
BASE and BASE-II participated in Lifebrain, a consortium of European studies funded by the EU Framework Programme Horizon 2020. Its goal was to harmonize, enrich, and fully exploit some of the largest longitudinal studies of age effects on brain, cognition, and mental health in Europe. Its focus was on the effect, over time, of education, socioeconomic status, and lifestyle factors such as sleep, physical activity, and diet on potentially enhancing and protecting brain structure and function. This consortium successfully ended in 2023 and published more than 80 papers in high-ranking journals regarding the effect of these factors on cognitive and mental health throughout life (see https://www.lifebrain.uio.no/publications/)
Key References
Lifebrain Researchers at the MPI for Human Development
| Andreas M. Brandmaier | Sandra Düzel |
| Maike M. Kleemeyer | Ylva Köhncke |
| Simone Kühn | Ulman Lindenberger |

